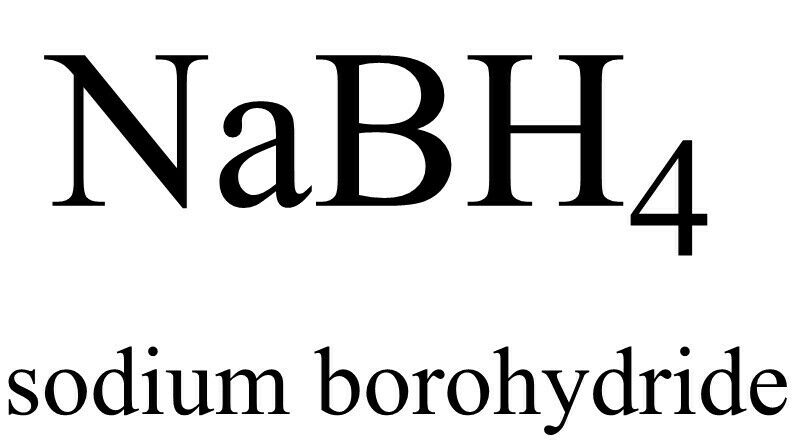-40%
Sodium Borohydride, NaBH4, (CAS: 16940-66-2), 98%, 100 g
$ 34.32
- Description
- Size Guide
Description
This is an updated version of our previous listing. Now we use an HDPE bottle with a pressure sensitive cap. This cap helps keep moisture out better than the f217 foam caps.The bottle is sealed with parafilm and vacuum sealed to protect the product from degradation.
We also included strips of parafilm and we were able to lower the price for you. Please let us know if we can improve your shopping experience.
Sodium Borohydride is a relatively weak reducing agent, and it is selective for ketones and aldehydes. However, there are many additives that can be combined with NaBH
4
to reduce other functional groups. Some combinations can even reduce esters and carboxylic acid. Complexes have been created that allow for enantioselective borohydride reductions. Even though NaBH
4
reacts with acidic, and neutral, water, and alcohols, ethanol and other alcohols are used as solvents for borohydride reductions. Sodium Borohydride can also be used for reductive animation however it will not reduce oximes by itself.
This reagent must be handled with care since it will react with protic solvents to release Hydrogen gas which can ignite.
Sodium Borohydride can react with acids to release diborane gas which is highly pyrophoric and poisonous. The reagent irritates the skin and respiratory tract. A thermal runaway reaction can occur when mixed with DMF. This reagent should only be handled in a fume hood with proper PPE. Review the MSDS before use. The seller accepts no liability for accidents in handling or use.